Our goal on this page is to provide clarity on many of the industry’s esoteric terms in order to create a more crisp interaction with our customers. The following terms are defined as we see and understand these industrial terms. The term definitions may appear differently elsewhere; keep in mind that these are our definitions and certainly not meant to be the last word on defining these industrial terms.
As always if you have comments or what you feel is a correction in regards to these definitions, please feel free to send us an email by clicking the “Contact Us” tab above and we will certainly consider your revision. Let’s get started.
Heat Sealers
The term heat sealer is merely a generic panacea term for any sealing device where high temperatures are present. It fails to narrow down any specific type of machinery, i.e. constant heat, impulse sealer, heat sealer, rotary sealer, etc. It’s merely a catch-all term for a machine designed to seal a thermal-formed type of pouch or container using temperature.
The following terms will more narrowly define the types of heat sealers under this term meant to describe a family of machinery.
Constant Heat Sealing
Constant heat sealing, as the name implies, refers to the process in which a machine is always hot and typically the film passes through a platen where it is cooled often in an ambient pressure or through a pinch roller, such as a rotary sealer. In the case of a rotary sealer, the film is conveyed past a constant heat platen and then through a secondary process is pinched as the pouch is shuttled through the pressure wheel and comes out the other side of the machinery sealed. There are a variety of constant heat machines. This machinery is typically not used for the sealing of medical pouches and bags as the cool down tier of medical packaging is typically important aspect in order to better control the process.
Impulse Sealing
Impulse sealing typically works as follows: A pouch is placed into the jaws of a sealing machine, and then the machine is invoked typically through a foot switch or a semi-automatic microprocessor controller. Once the jaws come together and the dynamic pressure is applied, the impulse sealer will heat, usually a nichrome wire or ribbon and it will pulse the voltage to the nicrom wire in order to maintain temperature or in the case of simple timer-driven sealers, will turn on the heating element for a predetermined time, and then stop heating. The system keeps the jaws and dynamic pressure applied to the film and the sealer will cool down the platen while still under clamping force. Once the predetermined temperature or cool dwell has been reached, the jaws will open and reveal a sealed bag. Impulse sealing is a preferred methodology for medical pouches and the sealing of medical bags as it allows quality control personnel to apply a repeatable standard to a process.
Rotary Sealing
Unlike impulse sealing where the pouch remains static during the packaging process, rotary sealing utilizes a conveyance system where the film is shuttled across a heated platen and pinch rollers impart pressure onto the flexible pouch in order to finish the bonding process. In the case of our MD-850 Medical Pouch Sealer that is also a rotary sealer, the system’s integrated transducer applies approximately 100 newtons of force as the neoprene wheel flattens and finishes off the bonding process. Although rotary sealing is certainly an exceptional way for higher through-put medical pouch processing, it does have limitations in that it will not bond all films. For instance, clear film to clear film or foil pouch would not be a good candidate for this process as many films do not accept being shuttled while they’re in a molten state. This process on the wrong film causes tug marks on the film that, in the case of medical device packaging, would create an unacceptable outcome. However for films such as 1070B TyVek to Mylar, the rotary sealer can be an exceptional choice. This medical pouch sealer has another advantage in that it can print important coding on the non-sterile side of the seal such as lot number, date of manufacturing, expiration dating, etc. There are other types of band and rotary sealers; some use hot air. But effectively they all work in a similar matter. But again it’s important to evaluate your pouch substraight in advance to determine if there may be an issue regarding your pouch and product under this methodology of medical pouch sealing.
Medical Pouch Sealing
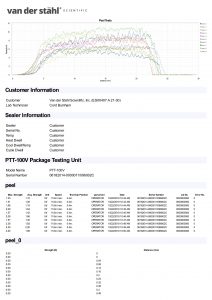
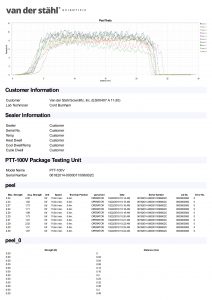
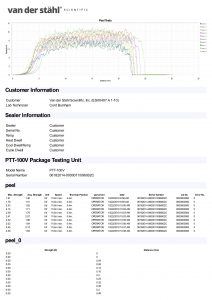
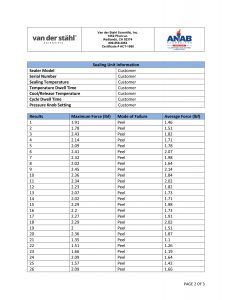
Medical pouch sealers or medical bag sealers typically use an impulse configuration as it allows quality control personnel to validate the process. In the process of validation, sometimes known as the IQOQPQ function, quality personnel can manage control attributes of the process which is pivotal to medical device package validation. The process again is typically impulse. An operator would place the medical pouch into the jaws of an impulse sealer and then activate the machine. The machine would create a specific temperature at the platen, would heat for a specific time, and then cool until a release temperature is met. The settings of the machine would be determined when the quality group has developed a design of experiment that identifies the best process settings or the best process attributes during the validation process. These numbers are typically developed during the OQPQ process and most companies are following the FDA’s QSR process as well as that of the ISO11607, which is a guideline for the packaging of terminally sterile pouches. The machinery utilized for medical packaging must have the ability to monitor and manage temperature at the sealing plate or heating element and they further must have alarm attributes that would disable the packaging process in the event that a critical parameter is unable to be met. It has been our experience that some medical device companies are using inadequate packaging machines, as they do not present with the basal requirements for medical device packaging. The packaging process just described is for flexible packaging and not for the packaging of lid tray stock. There are other systems for packaging medical device bags. A common medical pouch sealer is a rotary sealer that uses a constant heated platen. The medical pouch is fed in across the dual platens in the case of our MD-850 medical pouch sealer, and a pressured roller that is monitored by a pressure transducer pinches the molten film and TyVek together as it passes through the pressure system. The tertiary part of the packaging process of the MD-850 is to print lot numbers and date of manufacturing, date of expiration etc. on the TyVek non-sterile part of the pouch before the conveyance system deposits the sealed pouch at the other end of the unit. There are many different approaches to sealing a medical pouch and many of these are proprietary to the various companies that build the machinery. This description gives you a basic overview of the process, but certainly is not the only way that medical packages are sealed. If you have more specific questions regarding this process, we always invite you to contact technical support at 800-550-3854 and speak with a certified packaging engineer for additional clarity on this complicated process.
Vacuum Packaging
In the presence of moisture and ambient environment, many products such as food products, decay quickly and therefore vacuum packaging is often utilized to manage moisture and the O2 levels in the product’s environment. In some medical devices, particularly ones that include some type of chemistry or drug, it can be vital that moisture and O2 are not present in the pouched environment. In some cases the efficaciousness of the chemistry on the device is rendered ineffective if it is in the presence of moisture or oxygen. Medical pouch sealers such as our V-402, not only are fully validateable for a standard medical pouch but they also have the ability to vacuum package and then seal a bag that will be sterilized and sent to the point of use. Vacuuming the product can also have additional benefits such as stabilizing or cradling the device in a fixed position in order to thwart possible moving during the shipping process. Also some products that will not be packaged for the end user, such as sensitive optics that will be built up and stored for the end process of another finished device often can benefit by vacuuming the product to remain stable while in storage waiting for the next tier of manufacturing. Some medical pouch vacuum sealers also have the ability to monitor the amount of vacuum measured in torr or KPS. This allows medical device manufactures to set a protocol as to how much of a vacuum should be in place and to make the process repeatable. This is very important if as part of a product validation the quality group wishes to attach an SOP for exactly the amount of vacuum that should be invoked on a specific product and pouch. To learn more about vacuum packaging your product or vacuum packaging your medical device, contact technical support at 800-550-3054.
Gas Flush Packaging
As we discussed in the vacuum packaging segment, there are many benefits of packaging products that may decay in the presence of oxygen or moisture. Gas flushing a product typically means that an inert gas such as nitrogen is flushed into the pouch in order to displace the ambient environment which contains O2 and then vacuumed to pull out the bulk of the remaining gases in the pouch and then sealed. The thinking in gas flushing is that if there is any residual gas present within the pouched environment it would be in the form of an inert gas such as argon or nitrogen, rather than an ambient environment that may have destructive or moisture producing gases that could threaten the efficacious nature of the medical device or create spoilage on a product such as food.
Some pouches and product lend themselves to this process and others do not. It’s important to understand which products can benefit from the gas flush process. Many of our customers are so keen on removing any residual oxygen in the pouch, they will also as a final step install a small O2 scavenging desiccant. The thinking of course is that if there is any residual O2 left in the pouch environment, the scavenging desiccant can absorb the O2. This system is becoming very popular as it has a residual benefit, rather than just a passive benefit. To find out more about gas flush sealing contact technical support at 800-550-3854.





Recent Comments